Radical Reaction
Radical contain unpaired electrons:
You may recall that toward the start of Section 8 we said that the cleavage of H–Cl into H+ and Cl− is conceivable in arrangement simply because the particles that are framed are solvated: in the gas stage, the response is endothermic with ΔG = +1347 kJ mol−1, a worth so immense that regardless of whether the entire universe were made of vaporous HCl at 273 K, not a solitary atom would be separated into H+ and Cl− particles.
At temperatures above around 200 °C, nonetheless, HCl starts to dissociate, however not into particles. Rather than the chlorine particle taking both holding electrons with it, leaving an exposed proton, the electron pair framing the H–Cl bond is shared out between the two particles.
ΔG for this response is a significantly more sensible +431 kJ mol−1 and, at high gum based paint- tures (above around 200 °C, that is), HCl gas can be separated into H and Cl particles.

we presented the way that bromine extremists respond regioselectively with alkenes. Allow us to help you to remember one response you met at that point: revolutionary expansion to an alkene.
The item is an alkyl bromide, and is an alternate alkyl bromide from the one shaped when HBr adds to an alkene in an ionic way.
What does the peroxide do to change the component of the response?
Peroxides go through homolysis of the feeble O–O bond very effectively to shape two revolutionaries. We said that HCl in
the gas stage goes through homolysis in inclination to heterolysis: different sorts of bond are even
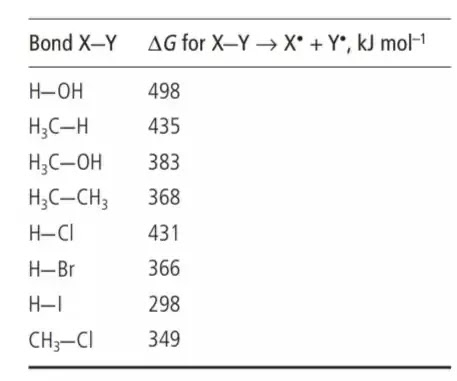
more vulnerable to homolysis. You can see this for yourself by seeing this table of bond separation energies (ΔG for X–Y → X• + Y•).
Dialkyl peroxides (dimethyl peroxide is appeared in the table) contain the exceptionally feeble O–O bond. The revolutionaries shaped by homolytic cleavage of these bonds, animated by a little warmth or light, start what we call an extreme chain response, which brings about the arrangement of the Br• radical, which add to the alkene's C=C twofold bonds.

Radicals structure by homolysis of weak bonds :
This is the main method of making radicals: unpairing a couple of electrons by homolysis,
making two new radicals. Temperatures of more than 200 °C will homolyse most bonds; on the other hand, some powerless bonds will go through homolysis at temperatures minimal above room temp erature.
Light is a potential fuel hotspot for the homolysis of bonds as well. Red light has
related with it 167 kJ mol−1; blue light has around 293 kJ mol−1. Bright (200 nm), with an
related energy of 586 kJ mol−1, will deteriorate numerous natural mixes (counting the
DNA in skin cells: sunbathers be careful!).
There are various mixes whose homolysis is especially imperative to scientific experts,
furthermore, the main ones are talked about thus beneath. They all have frail σ bonds, and
produce radicals that can be put to some synthetic use. The incandescent light are promptly homolysed by light, as should be obvious from the bond qualities in the table over, a reality that drives the extremist halogenation responses that we will talk about later.
Another compound that is regularly utilized in engineered responses for a similar explanation (despite the fact that it responds with an alternate arrangement of mixes) is AIBN (azobisisobutyronitrile).
Radicals form by abstraction:
Notice that we didn't put HBr on the rundown of particles that structure revolutionaries by homolysis: relative to the frail bonds we have been discussing, the H–Br bond is very solid (just probably as solid as a C–C bond). We see that how oxygen radicals dynamic hydrogen molecules from HBr. You may now prefer to contrast this system and comparable ionic responses.

Radicals :
Radicals can be produced by homolysis of powerless - bonds. Homolysis is affected by photochemical, warm or redox (electron move) strategies. A typical technique to start an extreme response is to warm a peroxide, for example, benzoyl peroxide or azobisisobutyronitrile (AIBN) 1.
The extremist ·C(CN)Me2 produced from AIBN is Or maybe lifeless, however is equipped for abstracting a hydrogen molecule from pitifully reinforced particles, for example, tributyltin hydride. The subsequent tributyltin extremist responds promptly with alkyl halides, selenides and different substrates to frame a carbon-focused Radicals.
Such engendering steps are delineated for decrease of a substrate RX . The attainability of this arrangement relies upon the overall response rates which themselves are prevent mined by the structures of the revolutionaries (counting that used to start the response).
In responses, for example, this, the trialkyltin extremist is in some cases alluded to as the chain transporter as it is constantly recovered to engender the cycle.
Example:

Radicals stability how to effect in Radicals formation ? Orders of Radicals Stability.
we utilized bond strength as a manual for the probability that bonds will be homolysed by warmth or light. Since bond energies give us a thought of the simplicity with which extremists can structure, they can likewise give us a thought of the dependability of those radicals whenever they have framed.
This is especially evident in the event that we think about the qualities of connections between similar molecules, for instance carbon and hydrogen, in various atoms; this table does this.
A couple of straightforward patterns are clear. For instance, C–H bonds decline in strength in R–H at the point when R goes from essential to optional to tertiary. Tertiary alkyl radicals are consequently the generally steady; methyl revolutionaries the most unstable.
C–H bonds close to forming gatherings, for example, allyl or benzyl are especially feeble, so allyl also, benzyl extremists are more steady. Yet, C–H bonds to alkynyl, alkenyl, or aryl bunches are solid.
Nearby practical gatherings seem to debilitate C–H bonds: radicals close to carbonyl, nitrile, or on the other hand ether practical gatherings, or fixated on a carbonyl carbon particle, are more steady than even tertiary alkyl Radicals.

Radicals structure by expansion:
The vital advance in the extreme expansion of HBr to an alkyne was the development of a radicals by extremist expansion. The Br• extremist (which, you will recall, was framed by abstraction of H• from HBr by RO•) adds to the alkene to give another, carbon-focused radicals. This is the extreme expansion instrument:
Similarly as charge should be moderated through a compound response, so should the turn of the elec- trons included. On the off chance that a reactant conveys an unpaired electron, at that point so should an item.
Expansion of a radicals to a turn combined atom consistently creates another extremist. Revolutionary expansion is there- front a second kind of revolutionary framing response.
The easiest revolutionary option responses happen when a solitary electron is added to a turn combined atom. This cycle is a decrease. You have just met a few instances of single- electron decreases: Birch decreases (Section 23) utilize the single electron framed when a bunch I metal (sodium, generally) is broken down in fluid smelling salts to decrease natural mixes.
Gathering I metals are regular wellsprings of single electrons: by surrendering their odd s electron they structure a stable M+ particle. They will give this electron to a few classes of particles, for example ketones can respond with sodium to shape ketyl revolutionaries.
Instructions to examine the structure of radicals: electron spin resonance:
For the last couple of pages we have been examining the species we call extremists without offering any proof that they really exist. Indeed, there is proof, and it comes from a spectroscopic strategy known as electron turn reverberation, or ESR (otherwise called EPR, electron paramagnetic reverberation). ESR confi rms that extremists do exist, yet it can likewise let us know a considerable amount about their structure.
Indeed, even the moderately straightforward range of the methyl extremist informs us a considerable amount concerning its structure. For instance, the size of the coupling steady aH demonstrates that the methyl revolutionary is planar; the trifl uoromethyl revolutionary is, then again, pyramidal. The oxygenated extremists •CH2OH and •CMe2OH lie some place in the middle. The computations that show this lie outside the extent of this book.
More knowledge:
1